Wavelength is a defining characteristic of any LED light. It determines the LED’s color (or invisibility in ultraviolet and infrared bands) and influences its efficiency and best applications. By selecting the right LED wavelength – from UV curing at 365 nm to SWIR imaging beyond 1500 nm – engineers can optimize performance for tasks ranging from sterilization to optical sensing.
 What Determines LED Wavelength?
What Determines LED Wavelength?
In LEDs, the wavelength of light emitted The wavelength light is fundamentally determined by the semiconductor material used in the LED. semiconductor’s band gap energy. Simply put, a light-emitting diode’s material composition sets the photon energy and thus its color or wavelength. The classic formula λ (nm) = 1240/Eg (eV) relates band gap (Eg) to emission wavelength (derivation and examples). Materials with larger band gaps emit shorter wavelengths (higher photon frequency), while smaller band gaps produce longer wavelengths (overview). For example, gallium nitride (GaN) with a ~3.4 eV band gap yields ultraviolet/violet light (~365–400 nm), whereas gallium arsenide (GaAs) at 1.4 eV emits in the near-infrared (~850–900 nm) (material-to-wavelength table). Intermediate compounds allow LEDs to span the visible spectrum: indium gallium phosphide (AlGaInP) covers red through yellow (~540–650 nm), and indium gallium nitride (InGaN) covers violet through green (~390–590 nm) (visible ranges). In summary, each LED’s wavelength is locked in by the semiconductor’s physics – a GaN blue LED cannot suddenly emit IR, and an IR LED’s chip cannot produce visible light because its band gap is too low (why band gap limits color).
How Light-Emitting Diodes Emit Different Spectra
Unlike lasers, LED light is not a single pure wavelength but a narrow band of wavelengths. LEDs are often called “quasi-monochromatic” sources. The output spectrum typically has a peak at the dominant wavelength and a full width at half maximum (FWHM) of a few tens of nanometers. For example, a typical red LED might have a peak at 630 nm and an FWHM ~20 nm, while a green LED might span ~30–40 nm in width. In general, LED emission bandwidths range on the order of 20–50 nm wavelength range can significantly affect the performance of light emitting diodes., much broader than a laser’s <1 nm linewidth but far narrower than a fluorescent lamp. According to a microscopy reference, a common 395 nm violet LED had a measured FWHM of only 14 nm. This relatively tight emission wavelength band is a result of the semiconductor physics – electrons recombine at energies mostly near the band gap, yielding a peaked spectrum. The upshot is that an LED provides a specific color of light without requiring optical filters (e.g., a 405 nm LED emits essentially only that violet light), which is extremely useful in applications like UV curing or biological excitation where a narrow spectral output is desired.
Understanding the Visible vs Invisible Spectrum (UV–IR)
The electromagnetic spectrum spans from high-energy ultraviolet to visible light to lower-energy infrared. By convention, ultraviolet radiation (UV) refers to wavelengths from about 100 nm up to 400 nm – none of which are visible to the human eye. This UV range includes UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm) sub-bands. The visible spectrum covers roughly 380 nm to 750 nm, encompassing violet, blue, green, yellow, and red light that human vision can perceive. Wavelengths determine color: for instance, blue light is around 450 nm, red light around 660 nm, and yellow LED light is around 590 nm. Beyond the red end, infrared light is any wavelength longer than about 700–780 nm. Infrared is subdivided into near-IR (approximately 0.7–1.4 μm), short-wave IR (SWIR, ~1.4–3 μm), mid-wave IR, and so on, extending to 1 mm. LEDs today can be engineered to emit in all of these regions except the very extreme ends. Standard indicator and lighting LEDs stick to the visible range (hence no IR or UV output in white LEDs), but specialized LEDs exist well into the UV and IR. It’s important to note that “invisible” UV or IR LEDs still obey the same physics – a UV-C LED at 265 nm emits higher-energy photons, while an IR LED at 940 nm emits lower-energy photons – the difference lies in the semiconductor materials used.
The Physics of nm: From 365 nm to 1750 nm
The range from ~365 nm (near UV) to ~1750 nm (SWIR) represents the span of most practical LED technologies today. At the short end, a 365 nm LED uses aluminum gallium nitride (AlGaN) alloy to achieve UV emission – these are used for UV curing lamps and have photon energy ~3.4 eV. Moving into the violet and blue (400–500 nm), indium gallium nitride (InGaN) LEDs dominate; this material system enabled the blue LED revolution and covers into the green (~530 nm) though efficiency drops in the “green gapFor longer wavelengths like true green (~555 nm) through red (~630–660 nm), aluminum gallium indium phosphide (AlGaInP) is utilized in various emitting diodes. A deep-red 660 nm LED, for example, is typically AlGaInP-based and around 1.9 eV band gap. Beyond red, in the near-infrared (780–1000 nm), LEDs often revert to gallium arsenide or related alloys. A classic 850 nm IR LED (used in remote controls or night-vision illuminators) is built on GaAs or GaAlAs and has a band gap ~1.4 eV. Pushing into the short-wave infrared, specialized materials like indium phosphide (InP) with indium gallium arsenide (InGaAs) can produce LED emission at 1300 nm, 1550 nm and even beyond. In fact, modern SWIR LEDs can reach ~1650 nm standard and even ~1750 nm in new designs. According to Tech-LED’s product data, their SWIR emitters cover roughly 1050 nm up to about 1650–1750 nm, spanning key sensing bands in the IR (typical peaks and options). Achieving light at 1750 nm with an emitting diode was once very difficult, but thanks to advanced InP epilayers and device structures, these longer wavelengths are now attainable (albeit at modest efficiency). Each “family” of LED wavelength thus corresponds to a different semiconductor technology, from AlGaN (UV) to GaN/InGaN (violet/blue/green) to AlGaInP (red/orange/yellow) to GaAs/InGaAs (IR). This also means the performance characteristics (efficiency, lifetime, output power) can vary widely across the spectrum, as we’ll compare next.
Comparing UV-C, Visible, and Infrared LED Ranges
How do LEDs in the ultraviolet, visible, and infrared ranges differ? There are several dimensions to consider: efficiency, lifetime, and challenges in design. In terms of efficiency, visible LEDs (particularly in the blue–green and red ranges) currently achieve the highest luminous efficacy and external quantum efficiency. By contrast, deep UV LEDs (UV-C) are notoriously less efficient – many have under 5% external quantum efficiency, meaning 95% of input power is lost as heat. This is due to the difficulty in creating high-quality wide-bandgap AlGaN materials and the lack of optimized UV emitter structures. Mid-range UV (UV-A at ~365–405 nm) is better – those LEDs can reach into double-digit efficiency and are widely used for UV curing and counterfeit detection. Visible LEDs (400–700 nm) are extremely efficient now; for example, a high-quality 450 nm royal blue or 530 nm green LED might convert 40–60% of electrical power into light. Red AlGaInP LEDs around 620–660 nm also achieve high radiative efficiency at low currents (though they suffer efficiency droop at high drive currents and are quite temperature-sensitive). In the NIR (near-infrared, 700–1000 nm), GaAs-based LEDs can be very efficient in radiometric terms (some 850 nm LEDs exceed 50% radiant efficiency, though since infrared is invisible, they have no “lumens”). As wavelengths extend further to SWIR (1000–1700 nm), LED efficiencies drop again – these photons are longer and materials like InP/InGaAs have more non-radiative losses. Nonetheless, steady progress is being made: for instance, state-of-the-art short-wave IR LEDs at 1550 nm now output tens of milliwatts of optical power, sufficient for many sensing applications.
Another contrast is in lifetime and degradation. UV-C LED devices (260–280 nm) tend to have shorter usable lifetimes and more rapid output decay compared to visible LEDs, partly due to higher defect densities in AlGaN and the harsh operating conditions (and they often require specialized packaging to avoid UV-induced degradation of encapsulants). Meanwhile, standard visible LEDs can easily last 20,000–50,000 hours (L70 lifespans), and even some UV-A LEDs approach similar longevity. IR LEDs often last long as well, but heat management is key – an overheated IR LED can suffer wavelength shifts and reduced output over time. Speaking of wavelength, one advantage of UV and visible LEDs is that their emission is inherently in-band (e.g., a 365 nm LED emits 365 nm light directly, unlike a mercury lamp that emits broad UV and visible lines). This means UV/visible LEDs don’t require filters to isolate a line – e.g., a 365 nm LED offers a narrow 365 nm output useful for fluorescence excitation. IR LEDs similarly emit in their intended band (e.g., 850 nm ± a small bandwidth). In summary, UV-C, visible, and IR LEDs each have their niches: UV-C excels at germicidal applications but currently has lower efficiency and higher cost; visible LEDs are the workhorse of lighting and displays with superb efficacy; IR LEDs enable sensing and illumination beyond human sight, with decent efficiency in the NIR and emerging capabilities in the SWIR.
Industrial Applications: Disinfection, Imaging, and Beyond
The choice of LED wavelength is often driven by the application, particularly in relation to color temperature. Let’s explore a few industrial use-cases across the UV–IR range:
- UV Disinfection and Curing: In water/air sterilization systems, UV-C LEDs around 265–280 nm are used to inactivate microbes by destroying DNA/RNA. In fact, 265 nm is known as the optimum germicidal wavelength for many pathogens. Today’s UV-C LED devices (typically 275 nm) are increasingly found in sanitizing wands, HVAC purifiers, and lab equipment. For UV curing of inks, coatings, and adhesives, UV-A LEDs in the 365 nm range are common – a 365 nm LED can efficiently initiate photopolymer reactions in adhesives and 3D printing resins. Additionally, there are violet 405 nm LEDs used in “antimicrobial” lighting – these aren’t true UV but a deep violet light that can kill certain bacteria over long exposures while remaining safe for occupied environments. Hospitals have begun installing 405 nm LED-based continuous disinfection fixtures to reduce surface bacteria.
- Visible Lighting and Indicators: Within the visible spectrum (400–700 nm), LEDs have revolutionized general illumination and signaling. High-power blue (450 nm) and “royal” violet LEDs combined with phosphors produce white light in everything from household bulbs to smartphone backlights. Specialty monochromatic visible LEDs serve in machine vision (e.g. 470 nm blue or 530 nm green lights to increase contrast of certain materials), in automotive lighting (amber ~590 nm LEDs for turn signals, red ~625 nm for brake lights), and in medical devices (e.g., 660 nm red light for pulse oximetry or therapy). An interesting niche is horticultural lighting: plants respond strongly to specific wavelengths. Deep red at 660 nm is highly efficient for photosynthesis, and The blue light color around ~450 nm is also crucial for various applications.. Hence, most LED grow lights use a mix of 450 nm and 660 nm LEDs to target chlorophyll absorption peaks. A 660 nm LED (coming soon) array gives the “blurple” glow you see in indoor farms, promoting flowering and growth in crops. These examples show how visible LED wavelengths are selected to match human or biological sensitivity curves – whether it’s our eyes or a chlorophyll molecule.
- Infrared Imaging and Sensing: In the IR realm, LEDs enable a host of sensing and imaging applications. For instance, most security and automotive night-vision cameras use IR LEDs at 850 nm as invisible floodlights – they illuminate the scene for the IR-sensitive camera while remaining mostly unseen by the human eye (850 nm has a faint red glow if you look directly). Some systems use 940 nm LEDs which are completely invisible (no red glow), though silicon-based cameras are slightly less sensitive at 940 nm than 850 nm. Beyond night vision, IR LED arrays are used in eye tracking (often ~810–870 nm) and in gesture sensors like those in gaming devices or AR headsets. Moving further, SWIR LEDs (short-wave infrared, 1050–1650+ nm) open new frontiers: machine vision in SWIR can inspect things regular cameras cannot. For example, moisture and certain plastics have distinct absorption in the 1400–1600 nm range – a SWIR LED at 1450 nm can help detect water content or find bruises in fruit on a sorting line. Likewise, 1550 nm LEDs are used in fiber-optic sensors and telecom test equipment (since 1550 nm is a standard fiber communications wavelength). A cutting-edge application is using a 1750 nm LED for resin curing detection on production lines (implementation examples) – essentially checking through plastic materials for proper curing or consistency, something not possible with shorter wavelengths. All these IR applications are enabled by picking LEDs with the right invisible wavelength to interact with the material or detector of interest.
- Beyond & Emerging Uses: The above are just a few examples. We also see ultraviolet LEDs being used in analytical instruments (e.g., 280 nm UV LEDs replacing deuterium lamps in spectrophotometers for protein analysis, with the benefit of narrow-band output and no warm-up). In medicine, specific LED wavelengths are being investigated for phototherapy – for instance, 405 nm and 470 nm LEDs for treating skin conditions or disinfecting wounds, and 660 nm deep red and 850 nm near-IR LEDs for photobiomodulation (claimed to assist in tissue healing and pain reduction). Each of these arises from the fact that biological molecules often have wavelength-specific responses: e.g., blue light kills certain bacteria, red/IR light penetrates tissue and can stimulate cellular activity. Thus, LED technology is enabling targeted light therapies that were not practical with broad-spectrum sources. As LED output powers continue to increase, we can expect even more industrial and medical processes to adopt wavelength-tailored LED solutions.
Selecting the Right Wavelength for Your Application
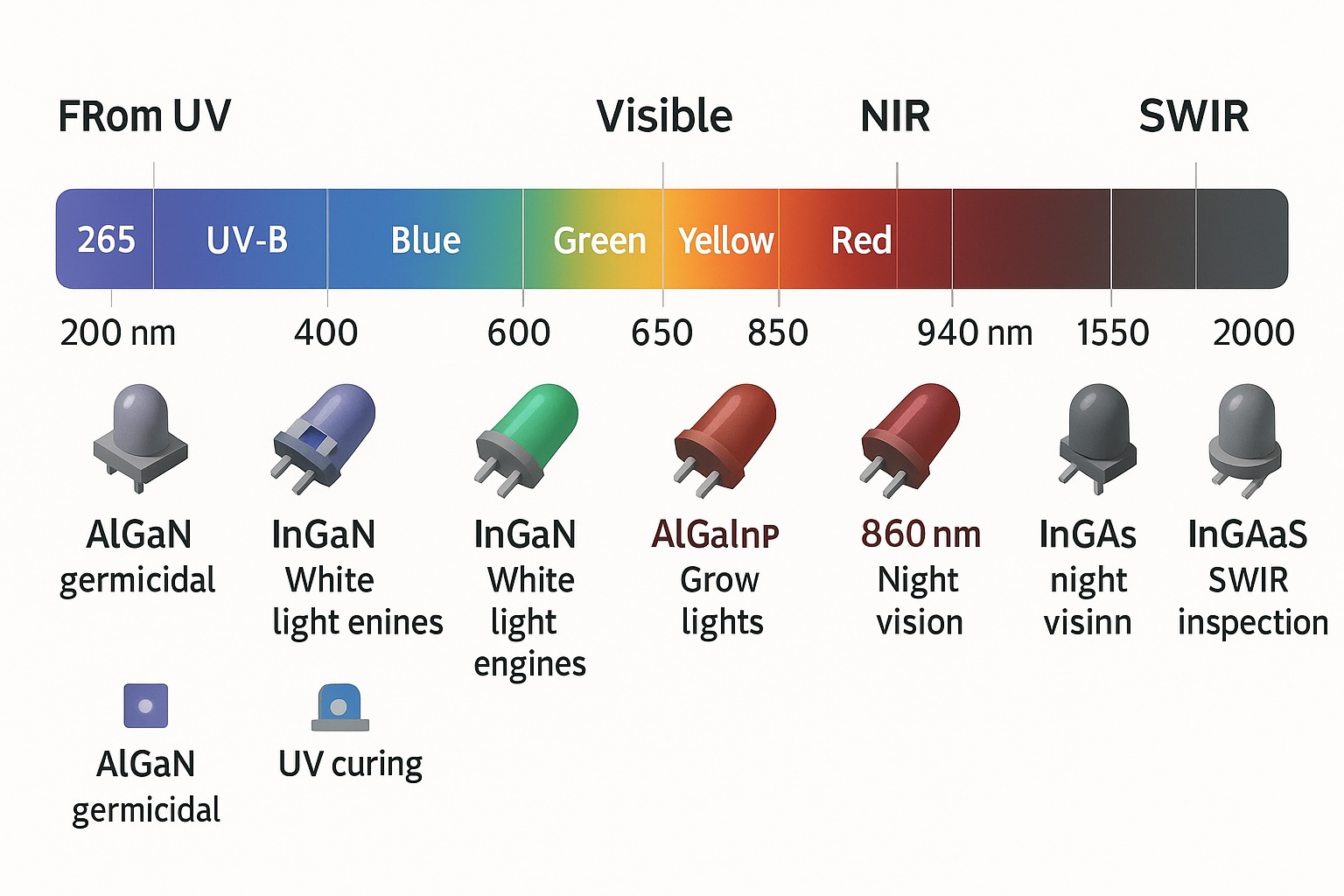
A full-spectrum map showing LED wavelengths from deep UV (265 nm) through visible light and into NIR/SWIR, with icons illustrating common semiconductor materials and industrial applications such as germicidal UV-C, UV curing, horticulture, night vision, and SWIR inspection.
How should a technical manager or engineer go about choosing the optimal LED wavelength? There are a few key considerations to ensure you get the best results:
- Match the Target’s Spectral Characteristics: First and foremost, identify what wavelength is most effective for the task. If you are curing a UV-sensitive adhesive, check its absorption spectrum – many adhesives cure best at 365 nm or 385 nm, so an LED in that range is ideal. For disinfection, as noted, ~265 nm UV-C is most germicidal. For plant growth, red 660 nm and blue ~450 nm are optimal. If your application involves a sensor (camera, photodiode, etc.), consider its spectral sensitivity: for example, a silicon photodiode is responsive up to ~1100 nm, so using an LED beyond that (say 1300 nm) would be ineffective unless you have a specialized detector. In short, know the absorption or sensitivity curve of whatever you are illuminating or sensing, and pick an LED that aligns with the peaks.
- Account for Human Factors and Safety: If the LED will be in an environment with people, consider whether its light is visible or poses any hazards. Infrared LEDs above ~900 nm are invisible, which is great for stealth illumination – but it also means users won’t know they’re on (no visible cue) and could stare into them unaware. UV LEDs, especially UV-C and UV-B, pose eye and skin hazards; these should be shielded or interlocked to prevent exposure. Even 405 nm “near-UV” can cause some eye discomfort with prolonged exposure, since it’s right at the edge of visibility. Conversely, if you need an indicator light that humans must see, obviously choose a visible wavelength light (e.g. LED light therapy). Don’t use an IR LED for a status indicator in the visible wavelength range! Human vision is most sensitive in green around 555 nm – but for warning lights, red (~630 nm) is often chosen for its high contrast. Thus, always factor in the end-user or environment.
- Consider Availability and Efficiency: Not all wavelengths are equally available in LED form. Manufacturers tend to offer standard LED wavelengths in certain increments (for instance, common bin wavelengths for high-power LEDs might be 365, 385, 395 nm in UV; 455 nm royal blue; 530 nm green; 590 nm amber; 625 nm red; 850 nm IR; 940 nm IR, etc.). If you need an unusual wavelength (say 510 nm teal or 720 nm deep red), you may have fewer product options or lower volume availability. It can sometimes be more practical to use a nearby standard wavelength unless the exact value is critical. Efficiency also varies with wavelength: the so-called “green gap” means mid-green LEDs around 555 nm are less efficient – if power output matters and you could use 530 nm or 590 nm instead, you might get significantly more light. Generally, the highest efficiencies are found in the ~450 nm blue and ~630 nm red regions, whereas far-red, deep-green, and UV LEDs have lower output. Weigh the trade-offs between getting the perfect wavelength versus the brightest one close to it.
- Mind Tolerances and Shifts: Remember that an LED’s specified peak wavelength is typically an approximate or typical value. There is a manufacturing tolerance – for example, one batch of “850 nm” IR LEDs might actually peak at 845 nm and another at 855 nm. If your application has a tight requirement (like it must be > 850 nm to avoid visible glow, or within a certain line for a sensor), you may need to spec a wavelength bin or do your own testing. Moreover, LED wavelength can shift with temperature and driving current. As an LED warms up, its peak can red-shift (move to longer nm). A rough rule is on the order of 0.2–0.3 nm per °C for many LEDs. So a device that is 850 nm at 25°C might be ~860 nm at 85°C junction temperature. If your system will see significant heating, choose a wavelength with some headroom or implement good thermal management to stabilize it. High-end applications sometimes include feedback or temperature control to keep LEDs at a stable wavelength if it’s critical.
- Evaluate Packaging and Optics: The LED wavelength you need might influence packaging choices and optical design. Deep UV LEDs, for instance, must be in quartz or ceramic packages because standard epoxy will yellow under UV. Similarly, some IR LEDs are packaged in clear or even black epoxy that filters visible light (so no red glow is seen). Consider how the LED will be integrated: do you need a lens on it for a narrow beam, or a diffused output? Different wavelengths interact with optics differently (e.g., short wavelengths scatter more in plastics). If using fiber coupling, ensure the fiber transmits that wavelength (many plastic optical fibers don’t transmit UV or >700 nm well). For multi-wavelength systems (say an RGB LED module or a combined UV + visible setup), be mindful that each wavelength may require its own drive current and thermal path. In summary, picking the wavelength is not just about the chip – it’s about the whole package and how the light is delivered to your target.
By taking into account these considerations – application match, safety, component availability, and system integration – you can confidently choose an LED wavelength that will deliver the best performance for your needs.
How LED Package Design Affects Wavelength Stability
Beyond the semiconductor material, the way a light emitting diode is packaged can influence its spectral behavior and stability. One major factor is thermal design. As discussed, when an LED’s junction temperature rises, its emission peak wavelength typically shifts to longer values (a phenomenon of band gap narrowing with heat). A well-designed package can help keep the LED cooler, thereby stabilizing its wavelength. For example, high-power LEDs often use substrates with high thermal conductivity (aluminum nitride ceramics, copper heat sinks, etc.) to draw heat away quickly. Advanced package styles like chip-on-board (COB) mount LED chips directly on a thermally conductive board, minimizing thermal resistance. In a COB module, many chips share one substrate and heatsink, which spreads heat efficiently. As a result, COB LEDs tend to have less wavelength drift under high drive, since the junction temperature is kept lower (thermal efficiency) and practical packaging notes. In contrast, an LED in a simple plastic through-hole package with poor heat sinking might see its wavelength wander more with current and ambient temperature swings.
Packaging can also affect the LED’s emitted spectrum through optical means. The encapsulation resin or lens, if not chosen for the wavelength, could absorb or shift some light. This is especially relevant in UV LEDs – they must use UV-transparent materials like quartz or specialized silicones; otherwise the packaging will absorb the UV and even discolor (which not only reduces output but can alter the effective spectrum over time). For infrared LEDs, packaging is generally silicone or epoxy which is transparent in the IR. However, consider that some LED packages include an optical filter to block visible light (for IR LEDs used in remote controls, a tinted epoxy that appears black filters out residual visible glow). That doesn’t change the peak wavelength, but it can improve the purity of the output as “perceived.” In multi-chip packages (like an RGB LED or a ²⁰ multi-die array), the design ensures each chip’s emissions don’t interfere with each other – for instance, phosphor-based LEDs (like white LEDs which use a blue chip plus phosphor) actually have a broader spectrum because the phosphor re-emits a range of longer wavelengths. If you needed a very narrow spectrum, you’d avoid any phosphor or wavelength-converting packaging.
Another angle is how packaging impacts long-term wavelength stability. LEDs can undergo “color shift” over their lifetime – for example, some white LEDs drift in color due to phosphor aging or silicone yellowing. For monochromatic LEDs, the peak wavelength might shift slightly after thousands of hours due to defects or material changes, especially if run hot. Choosing a package with stable materials and running the LED at a conservative drive current (or pulsed rather than continuous if possible) will mitigate such shifts. For critical uses, manufacturers sometimes spec the wavelength change over life (e.g., red LED might shift by <2 nm over 10,000 hours). If your application cannot tolerate that, you’d need to plan for periodic recalibration or replacement. Ultimately, a robust package – perhaps a metallized reflector, low-stress die attach, good encapsulant – works in tandem with the semiconductor to keep the LED operating at a steady wavelength and output. This is a good reason to source LEDs from reputable suppliers who optimize both chip and package. In summary, while the LED chip sets the nominal wavelength, the package design plays a pivotal role in maintaining that wavelength under real-world operating conditions.
Common Mistakes in Selecting LED Wavelengths
Even experienced professionals can slip up when choosing LED wavelengths. Here are some frequent mistakes and how to avoid them:
- Choosing the Wrong “UV” or “IR” LED: Not all UV or IR LEDs are equal. A common mistake is assuming a near-UV LED (say 400 nm) will disinfect like a UV-C LED – in reality 405 nm is visible violet and has very minimal germicidal effect compared to a 265 nm UV-C LED. Similarly, using a 850 nm IR LED when your sensor requires 940 nm (or vice versa) can drastically affect performance (many night-vision cameras are tuned to 850 nm and are less sensitive to 940 nm). Always differentiate UV-A vs UV-C, and specific IR bands, rather than generically saying “UV LED” or “IR LED” without the wavelength.
- Overlooking Wavelength Bandwidth: Users sometimes specify an “exact” wavelength (e.g., 520 nm green) without realizing the LED emits a range (~ ±15 nm). If your application has narrow spectral requirements (like exciting a precise fluorescence line), an LED’s ~30 nm bandwidth might excite unwanted frequencies. In those cases, either use an additional optical filter or consider a laser instead. Conversely, assuming an LED is as broad as a lamp and will cover a wide range is also a mistake – for instance, a 365 nm LED will not strongly emit 355 nm or 375 nm; it’s mostly within 10 nm of 365. Check the LED’s spectral width and ensure it suits your needs.
- Ignoring Tolerance and Bin Variations: As mentioned earlier, LED batches have wavelength bins. If you design something assuming a “450 nm” blue LED, the actual parts you get might be 445 nm or 455 nm. If your design is sensitive (e.g., an optical sensor with a filter), that could be an issue. It’s a mistake to not specify or at least test the tolerance. Work with the LED supplier to get a tight bin or adjust your design (for example, a photodiode with a wider acceptance band) to accommodate some variance.
- Using Human Perception for Invisible Light: Sometimes a design will include an IR LED and the team might mistakenly gauge its output by eye. For example, “we’ll know it’s working if we see it glow” – but 940 nm LEDs won’t visibly glow, and even 850 nm barely does. Another scenario: an UV LED can be on and you wouldn’t see it (except maybe a faint violet for 365–400 nm, below that it’s invisible), leading to safety issues or incorrect assumptions of it being off. Always use proper instruments (UV meter, IR camera, etc.) to verify invisible wavelengths, rather than human senses.
- Failing to Consider Environmental Effects: The deployed environment can alter effective wavelength. For instance, an LED’s peak can shift with temperature – if you calibrate a sensor with an LED at room temp but then use it outside in winter at -20°C, the LED might be a few nm shorter in wavelength, perhaps affecting a delicate measurement. Underwater applications must consider water’s absorption spectrum (red light is absorbed quickly in water, so using a 660 nm LED for an underwater beacon is a poor choice compared to, say, a 470 nm blue which travels farther in water). If the LED light is going into a high-temperature environment, its emission might red-shift and also broaden slightly, affecting the color temperature. All these external factors should be accounted for during selection and testing.
- Not Checking for Standards or Regulations: Some wavelengths fall under regulatory guidelines. For example, UV LEDs used in consumer products may need to comply with photobiological safety standards (risk group classifications). Certain IR wavelengths are used in biometric or medical devices that might need FDA clearance or eye-safety classification (especially if high power). It’s a mistake to pick a wavelength without ensuring you can legally and safely deploy it in the intended market. Also, from a standards perspective, some industries prefer certain standardized wavelengths (for example, 850 nm and 940 nm are standard for IR communication; deviating from these might cause compatibility problems).
Avoiding these pitfalls comes down to due diligence: read the LED datasheets carefully, understand the application requirements deeply, and if in doubt, consult with LED manufacturers or application engineers. Selecting the right wavelength LED is as much about the end-use context as it is about the LED specs on paper.
Future of Wavelength Engineering in Semiconductor LEDs
The landscape of LED wavelengths is continually expanding. Researchers and companies are pushing toward new frontiers in spectral coverage and control. One active area is the drive into ever-deeper UV. Current UV-C LEDs operate around 265–280 nm, but efforts are underway to achieve shorter wavelengths (e.g., 250 nm or even 222 nm “far UVC”) for specialized sterilization that might be safe for human exposure. These require advances in AlGaN materials and substrates – recent lab results have shown some success, but commercial devices at those wavelengths are still emerging. As efficiency improves in the UV-C band, we can expect much wider adoption of UV LED disinfection in healthcare and public spaces, replacing mercury lamps entirely.
Another frontier is the “green gap” and extending efficient LEDs to all colors without loss. The green gap refers to the drop in LED efficacy in the 530–570 nm wavelength range and the difficulty of making high-power, high-efficiency pure green/yellow LEDs. (background and research). To address this, researchers are exploring new material approaches, such as phosphide-nitride blends or even using quantum dots as down-converters to get those colors with less loss. There’s also interest in GaN-based red LEDs – normally red is done with AlGaInP, but those suffer from thermal sensitivity and can’t be driven as hard in high-power applications (like automotive brake lights in hot climates). If InGaN-based red LEDs become viable (some prototypes exist around 620 nm), it could unify the LED material system and significantly improve stability and lifetime for long-wavelength visible LEDs. Recent progress has indeed demonstrated lab devices of GaN red LEDs with acceptable performance, so in a few years we might see commercial offerings, effectively closing the green gap and offering a single platform (GaN-on-Si or GaN-on-Sapphire) for UV through red emitters.
On the infrared side, the future is bright (pun intended) for longer IR LEDs. Up until recently, LEDs beyond 1700 nm were more or less science fiction or extremely low output. But with companies announcing 1750 nm and even 2000 nm LEDs, we are entering the realm of mid-IR (though output is still modest). These open opportunities in gas sensing (where many gases have strong absorption in the 2–5 μm range) – while LEDs at 4 μm are not here yet, each incremental step in that direction (e.g., 2.6 μm LED reported by some research) could eventually lead to solid-state MIR illuminators for portable spectrometers or environmental sensors. The challenge is that traditional III-V LEDs struggle beyond ~1.8 μm because the materials become indirect bandgap or inefficient radiators. New approaches like “quantum cascade LEDs” (akin to quantum cascade lasers but as incoherent emitters) or novel alloys (like GaSb-based LEDs which can go to 2–3 μm) might help break this barrier. If achieved, we could see LEDs replace thermal sources in IR spectroscopy devices, giving instant-on, modulated sources at specific mid-IR lines for chemical analysis – no need for globars or lamps.
Another aspect of future LED wavelength engineering is spectral control and tunability. Today, an LED emits a fixed spectrum defined by its chip and any phosphors. But what if we want dynamically tunable wavelengths? One path is using multiple LEDs (as in an RGBW system for tunable white light or multispectral illuminators that mix several discrete LEDs to approximate a desired spectrum). We already have 4-in-1 and 5-in-1 LED packages for stage lighting that combine red, green, blue, amber, white, etc., to allow color tuning. We expect even more refined “spectral engineering” where LED arrays of many different narrow wavelengths (like 10 or 20 distinct LED colors) can be computer-controlled to sculpt the output spectrum for particular purposes (e.g., mimicking daylight variations for greenhouses or creating absorption-specific illumination for machine vision). On a single-chip level, there’s research into “quantum well engineering” that could yield multi-peak emission from one LED, or voltage-tunable emission (by switching which quantum wells are injecting). These are in early stages, but perhaps future LEDs could have two or three selectable wavelength peaks in one package.
Packaging advancements also influence the future of wavelength use. For instance, chip-scale LED packages and micro-LEDs are enabling new applications like LED-based displays (where each pixel is an LED of ~μm size). For those, having consistent wavelength bins (for color purity) is critical, and improvements in manufacturing are leading to tighter wavelength binning and less variation. We might reach a point where LED bins are so precise that for many uses you don’t need secondary optics or filters to get a desired narrow spectrum. Additionally, integration of LEDs with sensors (LED on one side, photodiode on the other in the same package) could create closed-loop systems that self-monitor wavelength and output, adjusting drive current to compensate for drift or aging. This kind of smart LED module would ensure a more stable effective wavelength output over time, enhancing LED light therapy applications.
In summary, the future will bring LEDs that are more efficient at the extremes (deep UV and deeper IR), more versatile in spectrum (with tunability and richer multi-wavelength combinations), and more stable and precise in their emitted wavelength. As semiconductor processes advance and as we possibly incorporate new materials (like two-dimensional semiconductors or perovskites, though the latter are more for emitters in other contexts), the phrase “any color you want” will truly apply. Engineers in the next decade may have an LED option for virtually every wavelength from 200 nm to 2000 nm and beyond, each at performance levels that make them viable for mainstream use. The continued evolution of LED wavelength engineering is set to further displace legacy light sources and unlock applications we haven’t even thought of yet – from more effective human-centric lighting that adjusts spectrum for circadian rhythms, to pocket spectrophotometers, to new medical diagnostics using specific light wavelengths. It’s an exciting spectrum of possibilities!
Key Takeaways on LED Wavelengths
A scientific plot comparing LED emission wavelength to semiconductor band gap energy, illustrating how materials such as AlGaN, GaN/InGaN, AlGaInP, GaAs, and InP/InGaAs determine ultraviolet, visible, infrared, and SWIR LED performance.
- Wavelength is tied to semiconductor material: The LED’s emission wavelength is dictated by its band gap. Different compound semiconductors (GaN, AlGaInP, InP, etc.) produce different color photons. This fundamental choice determines if an LED emits UV, visible, or IR (primer).
- LEDs are quasi-monochromatic: Unlike broad lamps, LEDs emit in a narrow band (typically 20–30 nm FWHM). They provide near single-color light without filtering, which is ideal for targeted applications (spectral characteristics).
- UV, visible, IR each have use-cases: UV-C LEDs (≈265 nm) enable germicidal systems, visible LEDs cover general lighting and displays, and IR LEDs (850 nm, 940 nm, SWIR, etc.) power remote sensing, night vision, and new machine vision techniques (applications).
- Application determines wavelength choice: Always select LED wavelength based on the target’s needs – whether matching a material’s absorption peak (e.g. 660 nm for plant chlorophyll) or a sensor’s sensitivity, or safety considerations (using 940 nm for an invisible beam, etc.). The right match maximizes efficiency and effectiveness.
- Packaging and design affect stability: Good thermal design (e.g. COB packages, proper heatsinking) keeps LEDs at stable wavelengths and output (thermal shift data). Poor cooling can lead to wavelength drift and lower light output over time. Choose packages suited for the wavelength (UV-resistant materials for UV LEDs, for example) to ensure longevity.
- The LED spectrum continues to expand: New LED innovations are pushing into deeper UV and farther IR. The once challenging “green gap” is closing and SWIR LEDs now reach ~1650–1750 nm. Future LEDs will offer even more wavelengths and possibly tunable spectra, giving engineers unprecedented control over the light we use.
By understanding these points, technical managers and engineers can make informed decisions in LED wavelength selection for any project. The right wavelength can dramatically improve system performance, whether it’s curing paint faster, capturing clearer images, or saving energy with the most efficient lighting.